In most technological applications, metals are used either in a finely divided form (e.g. supported metal catalysts) or in a massive, polycrystalline form (e.g. electrodes, mechanical fabrications).
At the microscopic level, most materials, with the notable exception of a few truly amorphous specimens, can be considered as a collection or aggregate of single crystal crystallites. The surface chemistry of the material as a whole is therefore crucially dependent upon the nature and type of surfaces exposed on these crystallites. In principle, therefore, we can understand the surface properties of any material if we
know the amount of each type of surface exposed , and
have detailed knowledge of the properties of each and every type of surface plane.
(This approach assumes that we can neglect the possible influence of crystal defects and solid state interfaces on the surface chemistry)
It is therefore vitally important that we can independently study different, well-defined surfaces. The most commonly employed technique, is to prepare macroscopic (i.e. size ~ cm) single crystals of metals and then to deliberately cut-them in a way which exposes a large area of the specific surface of interest.
Most metals only exist in one bulk structural form - the most common metallic crystal structures being :
For each of these crystal systems, there are in principle an infinite number of possible surfaces which can be exposed. In practice, however, only a limited number of planes (predominantly the so-called "low-index" surfaces) are found to exist in any significant amount and we can concentrate our attention on these surfaces. Furthermore, it is possible to predict the ideal atomic arrangement at a given surface of a particular metal by considering how the bulk structure is intersected by the surface. Firstly, however, we need to look in detail at the bulk crystal structures.
I. The hcp and fcc structures
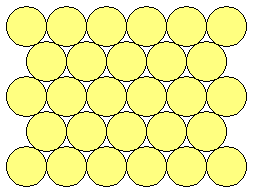
The hcp and fcc structures are closely related : they are both based upon stacking layers of atoms, where the atoms are arranged in a close-packed hexagonal manner within the individual layer.
The atoms of the next layer of the structure will preferentially sit in some of the hollows in the first layer - this gives the closest approach of atoms in the two layers and thereby maximizes the cohesive interaction.
When it comes to deciding where the next layer of atoms should be positioned there are two choices - these differ only in the relative positions of atoms in the 1st and 3rd layers.
Although it is not immediately obvious, the ..ABCABC.. packing sequence of the fcc structure gives rise to a three-dimensional structure with cubic symmetry ( hence the name ! ).
It is the cubic unit cell that is commonly used to illustrate this structure - but the fact that the origin of the structure lies in the packing of layers of hexagonal symmetry should not be forgotten.
(b) hcp structure
The ..ABABA.. packing sequence of the hcp structure gives rise to a three-dimensional unit cell structure whose symmetry is more immediately related to that of the hexagonally-close packed layers from which it is built, as illustrated in the diagram below.
II. The bcc structure
The bcc structure has very little in common with the fcc structure - except the cubic nature of the unit cell. Most importantly, it differs from the hcp and fcc structures in that it is not a close-packed structure.
At the microscopic level, most materials, with the notable exception of a few truly amorphous specimens, can be considered as a collection or aggregate of single crystal crystallites. The surface chemistry of the material as a whole is therefore crucially dependent upon the nature and type of surfaces exposed on these crystallites. In principle, therefore, we can understand the surface properties of any material if we
know the amount of each type of surface exposed , and
have detailed knowledge of the properties of each and every type of surface plane.
(This approach assumes that we can neglect the possible influence of crystal defects and solid state interfaces on the surface chemistry)
It is therefore vitally important that we can independently study different, well-defined surfaces. The most commonly employed technique, is to prepare macroscopic (i.e. size ~ cm) single crystals of metals and then to deliberately cut-them in a way which exposes a large area of the specific surface of interest.
Most metals only exist in one bulk structural form - the most common metallic crystal structures being :
For each of these crystal systems, there are in principle an infinite number of possible surfaces which can be exposed. In practice, however, only a limited number of planes (predominantly the so-called "low-index" surfaces) are found to exist in any significant amount and we can concentrate our attention on these surfaces. Furthermore, it is possible to predict the ideal atomic arrangement at a given surface of a particular metal by considering how the bulk structure is intersected by the surface. Firstly, however, we need to look in detail at the bulk crystal structures.
I. The hcp and fcc structures
The hcp and fcc structures are closely related : they are both based upon stacking layers of atoms, where the atoms are arranged in a close-packed hexagonal manner within the individual layer.
The atoms of the next layer of the structure will preferentially sit in some of the hollows in the first layer - this gives the closest approach of atoms in the two layers and thereby maximizes the cohesive interaction.
When it comes to deciding where the next layer of atoms should be positioned there are two choices - these differ only in the relative positions of atoms in the 1st and 3rd layers.
In the structure on the left the atoms of the 3rd layer sit directly above those in the 1st layer - this gives rise to the characteristic ..ABABA.. packing sequence of the hcp structure.
In the structure on the right the atoms of the 3rd layer are laterally offset from those in both the 1st and 2nd layers, and it is not until the 4th layer that the sequence begins to repeat. This is the ..ABCABC.. packing sequence of the fcc structure. Because of their common origin, both of these structures share common features :
- The atoms are close packed
- Each atom has 12 nearest neighbours ( i.e. a coordination number,CN = 12 )
Although it is not immediately obvious, the ..ABCABC.. packing sequence of the fcc structure gives rise to a three-dimensional structure with cubic symmetry ( hence the name ! ).
It is the cubic unit cell that is commonly used to illustrate this structure - but the fact that the origin of the structure lies in the packing of layers of hexagonal symmetry should not be forgotten.
(b) hcp structure
The ..ABABA.. packing sequence of the hcp structure gives rise to a three-dimensional unit cell structure whose symmetry is more immediately related to that of the hexagonally-close packed layers from which it is built, as illustrated in the diagram below.
II. The bcc structure
The bcc structure has very little in common with the fcc structure - except the cubic nature of the unit cell. Most importantly, it differs from the hcp and fcc structures in that it is not a close-packed structure.







Комментариев нет:
Отправить комментарий